The CRISPR Lie: Why 'Cellgorithm' Tech Won't Cure What They Promise (Yet)

Forget the hype. Deep analysis of CRISPR-based Cellgorithm technology reveals the hidden power struggle and true timeline for this cell programming revolution.
Key Takeaways
- •Cellgorithm shifts CRISPR from editing to complex biological 'programming' logic.
- •The immediate winners are IP holders consolidating control over platform technology, not necessarily patients.
- •Widespread, complex cures are still 10+ years away despite immediate clinical success claims.
- •High barriers to entry mean only large entities can afford to deploy this advanced cell programming.
The CRISPR Lie: Why 'Cellgorithm' Tech Won't Cure What They Promise (Yet)
The headlines scream revolution. We are told that CRISPR-based Cellgorithm technology heralds a new era of cell programming, promising precision medicine that reads like software code. But before we hand over our life savings to biotech futures, we must strip away the veneer of Silicon Valley optimism. This isn't just an incremental upgrade; it’s a fundamental shift in biological control, but the real story isn't the cure—it’s the gatekeepers.

The Unspoken Truth: Programming vs. Patching
What Cellgorithm truly represents is the maturation of genetic engineering from a blunt instrument (traditional CRISPR-Cas9) into a sophisticated logic gate. Traditional gene editing was like using a sledgehammer to fix a circuit board; Cellgorithm aims to write IF/THEN statements directly into the cell's operating system. This is powerful. However, the unspoken truth is that this level of cell programming demands an infrastructure that currently only a handful of mega-corporations can afford to build and validate. The winners here are not the patients waiting for treatments, but the patent holders controlling the foundational 'algorithms' that dictate cellular behavior.
We are seeing a massive consolidation of power. If you control the operating system for the next generation of therapeutics, you control the market. The promise of accessible, democratized gene editing remains distant when the tools themselves are locked behind prohibitive costs and complex regulatory hurdles. This isn't just about developing better gene therapy; it’s about creating the proprietary language of life itself.
The Economic Earthquake: Who Truly Wins?
The implications for the pharmaceutical industry are staggering. Existing drug pipelines built on small molecules or traditional biologics suddenly look antiquated. Companies that can master this new logic—this biological software—will render competitors obsolete. Think of it as the shift from steam power to microprocessors, but applied to human biology. While the public focuses on curing Type 1 Diabetes (a noble, long-term goal), the immediate economic win is securing the platform technology. Big Pharma isn't rushing to deploy cures; they are rushing to acquire the IP that makes future cures inevitable. This centralization of control over fundamental biological processes is a genuine economic threat to smaller innovators.
For context on the regulatory landscape surrounding these advances, look at how governing bodies are grappling with synthetic biology: Reuters on regulatory shifts.
Where Do We Go From Here? A Prediction
My prediction: Within five years, we will see the first highly publicized, successful clinical trial using Cellgorithm for a complex, multi-gene disorder—not cancer, but perhaps a rare metabolic condition. This success will trigger a massive, unprecedented influx of capital into companies owning the core intellectual property, essentially creating a 'blue chip' tier of biotech stocks based purely on their genetic coding capabilities. However, widespread, accessible treatments for common ailments like heart disease or Alzheimer's will remain elusive for at least a decade. Why? Because programming a cell to perform a complex task reliably across billions of patients in a chaotic in vivo environment is exponentially harder than demonstrating proof-of-concept in a petri dish. The hype cycle demands immediate cures; reality demands rigorous, slow validation.
The promise of CRISPR technology is real, but the path to mass application is paved with proprietary code and staggering development costs. Don't buy the cure yet; watch the patent wars.
Gallery

Frequently Asked Questions
What is the primary difference between traditional CRISPR and Cellgorithm technology?
Traditional CRISPR primarily acts as a precise molecular 'scissors' to cut or insert DNA. Cellgorithm technology integrates this capability into complex, programmable logic circuits within the cell, allowing for conditional responses (IF/THEN statements) rather than just static edits.
Why are critics skeptical about the immediate impact of this new gene therapy approach?
Skepticism stems from the immense complexity of translating in-vitro success to reliable in-vivo application across diverse human populations, coupled with the high cost and proprietary nature of the underlying programming IP.
How will this technology affect the current pharmaceutical industry?
It threatens to obsolete existing small-molecule drug development pipelines by offering a superior, software-like method for controlling biological outcomes, leading to massive M&A activity focused on platform acquisition.
Is this technology related to curing Type 1 Diabetes?
Yes, Type 1 Diabetes is a key target area for next-generation gene therapy, as it involves programming immune cells or pancreatic cells to function correctly, which aligns perfectly with the conditional control offered by Cellgorithm.
Related News
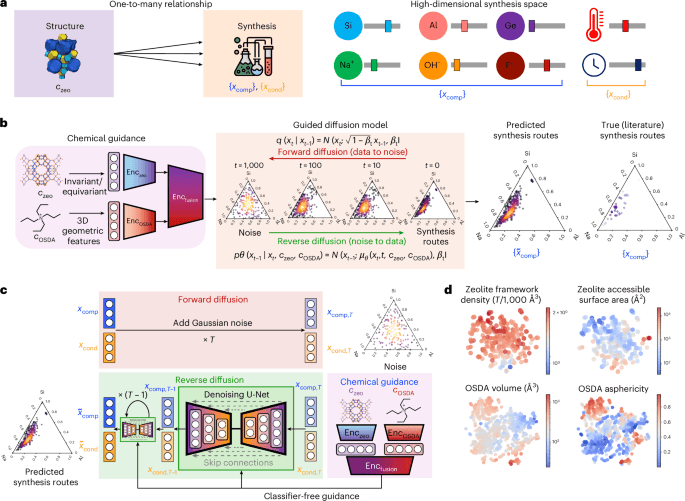
The AI Alchemy Revolution: Why DiffSyn Isn't Just New Science, It's a Threat to Traditional Chemistry Careers
Generative AI like DiffSyn is fundamentally reshaping materials science. Discover the unspoken winners and losers in this new era of chemical discovery.
The Hidden Cost of Lab-Grown Organs: Why Simplified Microfluidics Will Bankrupt Traditional Biotech
Digital microfluidic technology is changing 3D cell culture, but the real story is the centralization of pharmaceutical power it enables.

The Hidden Cost of 'AI Saviors': Why Ravender Pal Singh's Math Background Exposes Silicon Valley's Shallow Hype Cycle
The journey from pure mathematics to AI innovation isn't just career progression; it's a warning about AI safety. Unpacking the real stakes.

DailyWorld Editorial
AI-Assisted, Human-Reviewed
Reviewed By
DailyWorld Editorial